According to authors of a 2015 study, there were 2.5 million Americans living with hip replacement implants in 2010.(2) This number is expected to continue to rise, with potentially hundreds of thousands of people seeking hip replacement surgery annually.(3)
On one hand, the increase in hip replacement surgery seems like good news. The numbers suggest people are living longer, more active lives. Growing numbers of people want to continue to remain active into their 50s and beyond.(4)
Baby boomers are a good example. As they age, the rates of diagnosis and treatment of advanced arthritis are on the rise. With this increase comes a “growing demand for increased mobility and quality of life.”(5)
The downside to this growing demand is that manufacturers of hip replacement implants may rush to introduce new hip implants to the market before they are properly evaluated for safety and efficacy.
Hip Implant Litigation
After undergoing faulty hip procedures and suffering serious hip replacement complications related to their hip devices, a number of people have come forward seeking compensation from medical device manufacturers.
Weitz & Luxenberg and other law firms have been quick to respond to the needs of clients to act against manufacturers of faulty hip replacement implants. In several of the verdicts, juries have sided with the recipients of defective hip devices, awarding large sums to victims of faulty implants. In other instances, manufacturers have agreed to settle rather than risk potentially larger penalties in subsequent jury trials. Examples of settlements include:
- Wright Medical — Wright Medical Technology Agrees To $240M Hip Implant Deal(6)
- Stryker — Stryker Adds Patients To $1B Hip Implant Settlement (7)
Weitz & Luxenberg is privileged to count Practice Group Chair Ellen Relkin as one of our skilled and talented attorneys. Ms. Relkin has played an integral role in securing verdicts and settlements on behalf of people harmed by defective hip implants across the country. She has been the court-appointed lead counsel in many of these litigations.
Weitz & Luxenberg is continuing to take legal action against medical manufacturers through hip implant litigations. If you underwent a revision, or corrective, surgical procedure because of complications linked to hip replacement surgery, we want to hear from you. If a doctor has informed you that you have elevated levels of cobalt and chromium in your blood as the result of a hip implant, we want to hear from you.
Our experienced teams of Weitz & Luxenberg attorneys have worked on successful lawsuits for defective hip replacement implants and can help you evaluate your legal options. We invite you to complete this online form to contact us for a free consultation or call us at (833) 977-3437.
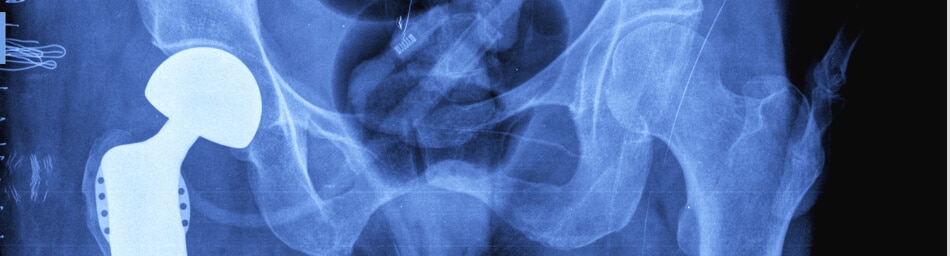
Ongoing Hip Replacement Lawsuits
While some medical device manufacturers already have had their “day in court,” other medical device manufacturers, such as Smith & Nephew, continue to face multiple hip replacement lawsuits in multidistrict litigation.(8) Multidistrict litigation allows many similar suits, in these cases defective hip replacements, to be combined for pretrial discovery and representative bellwether trials.
Weitz & Luxenberg is currently accepting clients who received faulty Smith & Nephew Modular SMF, Modular REDAPT, or EMPERION hips during hip replacement procedures and have had to undergo revision or are experiencing certain complications as a result of their implanted devices.
In addition to certain Smith & Nephew hips implants, we are also helping people who have had trouble with these other manufacturers’ hip replacement devices:
- Wright Medical Conserve
- Stryker LFIT V40, Rejuvenate, ABG II, Accolade, Citation or Meridian
- OMNIlife science Apex K2
If you had to undergo a hip implant revision surgery because of medical complications, have been advised to undergo a revision implant procedure, or are experiencing severe pain or other debilitating symptoms as a result of metal-related complications or implant fracturing, we urge you to contact us immediately.
Components of metal-on-metal hips may contain the metals chromium and cobalt. These metals have the potential to damage surrounding tissue when the components rub against each other. The friction can wear the surfaces down and metal particles can then be released and absorbed into neighboring tissues.(9)
Get a free consultation and more information about your legal options, please contact us today.
Get a Free Case ReviewPotential metal-related implant complications may include:(10)
- Metallosis — accumulation of tiny metal particles in tissue surrounding the implant due to corrosion of the implant
- Damage to tissue around the implant — such as tissue death (necrosis) or aseptic lymphocytic vasculitis associated lesions (ALVAL)
- Osteolysis — the disappearance or destruction of bone tissue
- Pseudotumors — masses in the soft tissue
The Smith & Nephew EMPERION modular hip system has been linked to implant stem fracturing or breakage. If either complication occurs, you could potentially require a revision surgery and replacement of the implant. The Australian Joint Registry identified the Emperion as having twice the rate of revision as other primary total hip replacements.(11) (12) (13) (14)
If you have experienced severe medical complications, including revision surgery, related to a faulty hip implant, we urge you to contact Weitz & Luxenberg immediately. One of our attorneys with experience with defective hip replacement devices can help you consider possible legal courses of action.
Hip Implant Recalls
In recent years, each of these manufacturers has initiated hip implant recalls:
- DePuy Orthopaedics(15)
- Wright Medical(16) (17)
- Stryker Corporation(18) (19)
- Smith & Nephew(20) (21) (22)
For the latest information about hip implant recalls, as well as other medical devices, you can visit the U.S. Food and Drug Administration (FDA) medical device recall database.
The “FDA uses the term ‘recall’ when a manufacturer takes a correction or removal action to address a problem with a medical device that violates FDA law.”(23) Manufacturers may recall a medical device when it is defective, when it poses a potential risk to people’s health, or both, which all of these manufacturers have done. The FDA may also initiate a recall if a device manufacturer refuses recall a product linked with “significant health problems or death.”(24)
The FDA categorizes recalls based on a medical device’s risk potential. For example, a Class 1 recall is the most serious. In these instances, there is a “reasonable probability” using or being exposed to the defective device will “cause serious adverse health consequences or death.”(25)
A Class 2 medical device recall involves “a situation in which” using or being exposed to the defective device could “cause temporary or medically reversible adverse health consequences.”(26)
A Class 3 recall means the device is defective but “is not likely to cause adverse health consequences.”(27)

Hip Replacement Implants
Nearly 40 years ago, surgeons performed the first FDA-approved total hip replacement surgery(28). The implant inserted at that time “had a smooth, polished stainless steel stem and a plastic socket. Both pieces were cemented or glued into place.”(29)
We would feel privileged to assist you. For a free consultation and more information about your legal options, please contact us today.
(833) 977-3437Patients considering having a hip replacement surgery back then had few choices of hip implants available to them.(30) Today, many more hip replacement options exist.
If you perform an Internet search, you discover a number of manufacturers offering a variety of hip replacement devices. According to the School of Medicine and Public Health at the University of Wisconsin-Madison, however, “extensive joint-replacement advertising” is targeting “younger patients with promises of restored function.”(31)
Restored function to a worn hip joint may be a good thing, particularly for those with debilitating osteoarthritis, “the most common reason” people undergo hip replacement surgery.(32)
“While it’s true that procedures like resurfacing have made hip…replacements more attractive to an even wider pool of patients,” the reality is that major joint replacement surgery is not routine and free of drawbacks.(33)
Faulty Hip Procedures
Faulty hip implants can be devastating. The “long-term ramifications of a failed joint replacement — decreased function, less pain relief and extended recovery time — can have a significant effect on a patient’s quality of life. And every hip-revision surgery increases the chances of complications.”(34)
Some degree of pain, immobility, and inconvenience can be expected following a hip replacement procedure. However, extreme or severe ongoing complications should not be taken lightly.
If you have undergone a hip replacement revision, or corrective surgery, or if you have suffered extreme medical complications following a hip implant procedure, we urge you to contact Weitz & Luxenberg. One of our attorneys will be happy to provide you with information and guidance about your legal options.
As a national law firm with more than 30 years of experience in handling large-scale defective medical device lawsuits. Weitz & Luxenberg attorneys have represented thousands of clients over years, and our firm has a history of winning. We would feel privileged to assist you.
For a free consultation, you may call us at (833) 977-3437 or complete the form on this web page, and one of our attorneys will contact you shortly.
Although a past record does not guarantee future success, Weitz & Luxenberg has the experience and resources necessary to stand up to the large manufacturers of defective medical devices.
