We are no longer accepting new cases.
Some patients who have received either the Zimmer M/L Taper Hip or M/L Taper Hip with Kinectiv, along with a Zimmer VerSys Femoral Head, have experienced metallosis and other metal-related problems. Injuries specifically seem to occur when these Zimmer taper hip implant parts are paired with the Zimmer VerSys Femoral Head hip implant part.
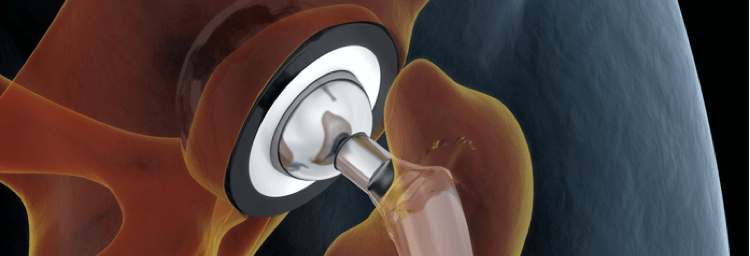
The titanium alloy of the Zimmer hip taper portion of the implant and the cobalt-chromium alloy of the VerSys head portion of the implant moving against each other may result in corrosion and the leaching of metal particles into surrounding tissues, leading to medical complications.
What Is the Zimmer M/L Taper Hip, M/L Taper with Kinectiv Technology and VerSys Femoral Head?
Zimmer’s M/L Taper Hip, M/L Taper Hip with Kinectiv Technology, and VerSys Femoral Head are types of hip implants. The M/L Taper and M/L Taper with Kinectiv Technology are implanted into the top of the thighbone. The thighbone is called the femur. The VerSys Femoral Head replaces the ball of the femur. (1) (2)
Why Patients Are Filing Lawsuits
Some patients are filing lawsuits against the manufacturer, Zimmer Biomet Inc., because they have experienced metal-related injuries and complications they are claiming are linked to their hip implant components. The metal materials used in Zimmer’s M/L Taper Hip and M/L Taper Hip with Kinectiv Technology are made with a titanium alloy. Zimmer’s VerSys Femoral Head is made of a cobalt-chromium alloy. Patients who have filed cases are contending “the interaction (junction) between the titanium alloy M/L Taper or Kinectiv and the cobalt-chromium alloy VerSys Head can result in trunnionosis (wear of the femoral head-neck interface), corrosion, and release of metal debris…” (3) (4)
As a result, some patients are alleging in lawsuits they have experienced serious medical complications including: (5)
- Metallosis.
- Adverse local tissue reaction.
- Loss of bone tissue (osteolysis).
- Other corrosion-related complications requiring revision surgery.
Brendan A. McDonough, Associate Attorney

Brendan A. McDonough works in Weitz & Luxenberg’s Drug and Medical Device Litigation practice group.. He plays a leading role in the firm’s efforts against Philips Respironics to hold the company responsible for injuries caused by their defective CPAP and BiPAP sleep apnea machines, which were recalled in June 2021.
Reporting Adverse Events
Patients who experience problems after undergoing hip replacement surgery can report these adverse events. The U.S. Food and Drug Administration (FDA) maintains a database of reported incidents.
This database is called the MAUDE, or Manufacturer and User Facility Device Experience database. You can search the MAUDE database to see if other people have reported problems with Zimmer M/L Taper Hips and the VerSys Femoral Head.

Problems with Some Zimmer Hip Models Can Be Serious
Not everyone who experiences problems with their hip implant reports adverse events to the FDA. In at least one instance, a patient experienced severe problems with her Zimmer M/L Taper stem with a Kinectiv Technology modular neck with a taper modular head-neck junction. This complication was not reported to the MAUDE database. (6)
The patient had undergone a routine total hip replacement procedure. Just two years after having surgery, she started experiencing increasing pain in her groin along with a limp. Lab tests showed she had high levels of metal in her blood. (7)
Her knee function was severely impaired such that she needed to undergo a revision, or corrective, surgery. During surgery, doctors found a large cyst, a type of pseudotumor. Doctors also found dead muscle tissue and other damage to the woman’s local tissues surrounding the implant (8)
Parts of the implants themselves also showed severe damage. Some metal areas of the implant, specifically those around the head and modular neck, had corroded. (9)
Cases Combined in Multidistrict Litigation
In the fall of 2018, multiple cases involving the Zimmer M/L Taper Hip Prosthesis or M/L Taper Hip Prosthesis with Kinectiv Technology combined with the VerSys Femoral Head were combined in a multidistrict litigation. (10)
They were assigned to the Honorable Paul A. Crotty in the Southern District of New York. (11)
When lawsuits “involve common questions of fact,” centralization of multiple lawsuits can be more efficient than handling the cases separately. (12)
What You Can Do if You’re Having Issues with Your Zimmer Hip Implant.
Weitz & Luxenberg is currently accepting cases involving Zimmer’s M/L Taper Hip or M/L Taper Hip with Kinectiv, combined with Zimmer’s VerSys Femoral Head. If you have experienced significant medical complications due to your Zimmer hip implant, we invite you to contact us. Our experienced attorneys can provide you with more information regarding your legal options.
We may be able to help you get compensation for your pain and suffering. Patients who had revision surgery due to metallosis-related complications, or who need a revision surgery but are unable to have one due to medical reasons, should contact us.
We are prepared to help you. For a free consultation, we encourage you to call us at (833) 977-3437 or you may complete the form on this web page. One of our client relations representatives will contact you shortly.