UPDATE: In August 2022, Exactech sent out notification indicating non-crosslinked liners were also included in an expanded recall. The ultra-high molecular weight polyethylene acetabular liners were packaged in non-conforming vacuum bags. This includes ACUMATCH, MCS, and NOVATION liners and affects implants done since 2004.
Exactech Issues Recall
In the summer of 2021, the manufacturer issued a recall for some models of these liners. The reason Exactech gave was “Risk of edge-loading and premature prosthesis wear is possible in a specific subset of patients with certain implant configurations and surgical implant positioning.” (1)
Symptoms of medical complications resulting from Exactech Connexion GXL liner wear may include:
- Pain.
- Stiffness.
- Limited mobility.
- Loosening.
- Osteolysis — disappearance or degeneration of bone tissue.
- Revision surgery due to device failure.
In June 2022, Weitz & Luxenberg attorneys filed a petition to create a multidistrict litigation (MDL) for these Exactech defective polyethylene inserts. It was approved on October 7, 2022 by the Judicial Panel on Multidistrict Litigation. The MDL covers liners for the hip, knee, and ankle. It is called In re: Exactech Polyethylene Orthopedic Products Liability Litigation in the Eastern District of New York in Brooklyn and is MDL No. 3044. Two W&L attorneys were appointed to leadership positions in the MDL. Ellen Relkin, partner and chair of our Drug and Medical Device Litigation team, is serving as co-lead counsel. Attorney Danielle Gold is a member of the Science and Experts subcommittee.
What Is the Exactech Connexion GXL Acetabular Liner?
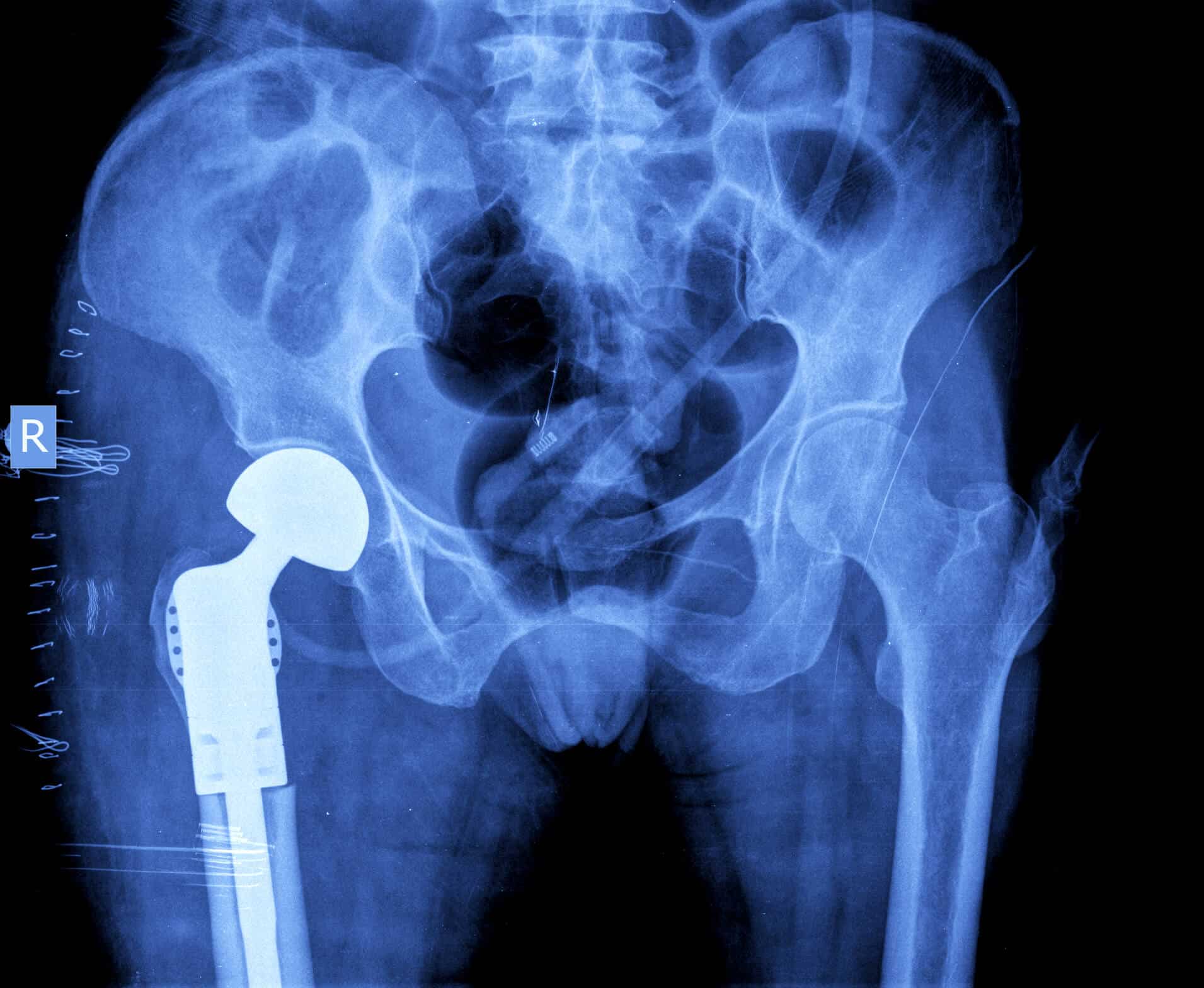
All hip replacements contain several different parts, often including an acetabular liner. When a person’s hip is replaced, typically a metal cup is inserted into the hip socket (acetabulum), often anchored with screws.
An acetabular liner, typically made of some form of plastic or ceramic, is often inserted into this acetabular cup. A femoral head component — a spherical piece of metal intended to replicate the top or “ball” of a person’s femur bone — sits against the acetabular liner.
The Exactech Connexion GXL acetabular liner is intended for use with other Exactech hip components implanted during total hip replacement.
For a free consultation and more information about your legal options, please contact us today.
(833) 977-3437Studies Raise Concerns About Exactech Connexion GXL Hip Implant Liners
At least two recently published studies have reported earlier than expected failure of the Exactech Connexion GXL acetabular liners due to wear.
Early Failure of Implant
Orthopedic surgeons at the Department of Orthopedics and Rehabilitation at the University of Florida College of Medicine authored one recently published study discussing early failure of the Exactech Connexion GXL liners. The surgeons reviewed their institutional database and identified problems and failures due to wear. (2)
These physicians reviewed patient data from January 2009 to June 2019 for all patients who had revision surgery after being implanted with the Exactech Connexion GXL acetabular liners at their institution. These orthopedic surgeons found that all of the patients who had to have a revision surgery, or revision surgery had been recommended, after being implanted with an Exactech Connexion GXL acetabular liner demonstrated radiographic osteolysis around the liners. This means X-rays of their hips showed bone loss and destruction to the hip socket (acetabulum). (3)
The researchers expressed concern that the Exactech Connexion GXL liner could be prone to early failure. Patients included in this study were diagnosed with hip implant failure after having their original hip implant surgery, on average, less than five years earlier, “a timeframe that is uncommon in technically well-done” total hip replacement.
FDA Adverse Events
The authors also performed a U.S. Food and Drug Administration (FDA) Manufacturer and User Facility Device Experience (MAUDE) database review of the Exactech Connexion GXL liners, to determine if adverse event reports had been submitted to the FDA in connection with these hip components. This MAUDE database review yielded 83 patients who had undergone revision after being implanted with an Exactech Connexion GXL liner.
There were 22 of these patients specifically noted as having revision due to “poly wear” or “osteolysis.” When reported for these adverse events, the average time between the Exactech Connexion GXL liner’s initial implantation to revision surgery was approximately 6.5 years.
These surgeons recommended that doctors keep a close eye on patients who have been implanted with these hip implant liners. (4)
Wear and Osteolysis
Another group of orthopedic surgeons, this time from the Hospital for Special Surgery in New York, authored a study published a few months later in the journal Arthroplasty Today. The study discussed “catastrophic early polyethylene wear demonstrat[ing] a concerning trend with the use of the Exactech Connexion GXL liner.” These physicians noted that the Exactech Connexion GXL liner seemed linked to “severe polyethylene wear and osteolysis” again occurring, on average, within just 5 years of the patients’ index surgery. (5)
The patients included in the study showed signs of “catastrophic early polyethylene wear” seen on radiographs and “grossly visible and palpable wear” on the Exactech Connexion GXL acetabular liners. These liners were retrieved during revision surgery with acetabular cups and femoral stem components that were “clinically and radiographically well-fixed.” The authors of this study said this “unusual early excessive polyethylene wear” warranted “further investigation…to evaluate material characteristics which may have caused this accelerated wear.” (6)
The authors of both studies contemplated potential design flaws of the Exactech Connexion GXL acetabular liners that may be causing these problems with early wear.
W&L Can Help People Injured by Exactech Connexion GXL Hip Implant Liners
If you or anyone you know has experienced complications after a hip replacement that used an Exactech Connexion GXL hip liner, contact us for a free case evaluation.
Get a Free Case ReviewWeitz & Luxenberg would like to hear from anyone who has experienced serious complications after undergoing a hip replacement procedure in which they were implanted with an Exactech Connexion GXL hip implant liner.
Specifically, we urge anyone who has been advised to undergo a revision surgery or has already undergone a revision surgery because of complications linked to the Exactech Connexion GXL hip implant liners to contact us for more information.
Patients and their families may be entitled to compensation, not only for any related medical expenses but also for lost wages and other costs. One of our experienced attorneys can help you consider your legal options.
Weitz & Luxenberg invites you to contact us for a free consultation. Please call (833) 977-3437 or use the online form on this web page to reach out to us. We can help.