Smith & Nephew Removes Neck Component from Market
On November 15, 2016, the company announced a “voluntary market removal” of the modular neck component commonly used in its Modular SMF and Modular REDAPT Revision models due to what the company called “a higher than anticipated complaint and adverse event trend.” Monolithic versions of the SMF and REDAPT Revision Femoral Hip Systems were not affected.
As the company said in its Urgent Field Safety Notice, “In the worst case scenario implanted patients are symptomatic and exhibits [sic] adverse tissue reaction to metal debris which may lead to revision surgery.”(2)
For a free consultation and more information about your legal options, please contact us today.
Get a Free Case ReviewIn the associated Dear Doctor letter accompanying the notice, the UK-based company wrote that based on an analysis of available data sets, “patients implanted with the modular neck hip prostheses may be at greater risk of revision surgery than with comparable monolithic products.”
Weitz & Luxenberg is now accepting cases of people who have Modular SMF or Modular REDAPT hips and have had subsequent surgery to have their hips revised, have been advised they may need revision, or are experiencing pain or other symptoms indicative of metal-related complications due to their hips.
If you’ve undergone revision surgery or if your doctor has advised you to have revision surgery because of metal-related problems with either of these hips, you may want to consult with an attorney as to whether you should seek compensation for medical and other expenses.
Modular Hip Implant Complications
The modular versions of the SMF, originally approved and marketed as the MIS Hip Stem in 2008, and REDAPT Revision implant, approved by the FDA in 2012, each have cobalt and chromium neck pieces that fit into titanium stems.
In a letter to doctors notifying them of the voluntary market removal, Smith & Nephew suggested that physicians should consider additional follow-up apart from routine protocols for patients who are exhibiting symptoms of metal-related complications with Modular SMF and Modular REDAPT Revision Femoral Hip Systems.
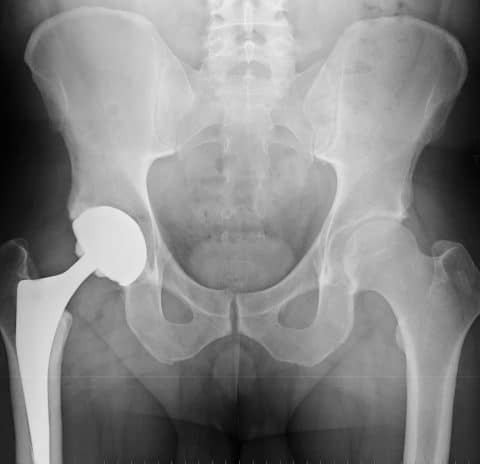
These additional follow-up measures for symptomatic patients should be determined on an individual case-by-case basis following assessment of clinical circumstances, and could include blood testing to check for elevated cobalt and chromium levels, and when appropriate, the use of ultrasound or other imaging to check the health of patients’ soft tissue around the implants.(3)
In some cases, the friction of the metal-on-metal components rubbing against each other can cause metal ions in the components to rub off and be absorbed into the body, potentially causing serious medical complications.(4)
Smith & Nephew as the manufacturer of the Modular SMF and Modular REDAPT Revision devices suggests annual examinations for patients exhibiting symptoms of metal-related complications. The company also recommends that a higher frequency of monitoring blood metal ion levels, such as every three months, might be considered for patients whose blood ion levels rise above 7 ppb.(5)
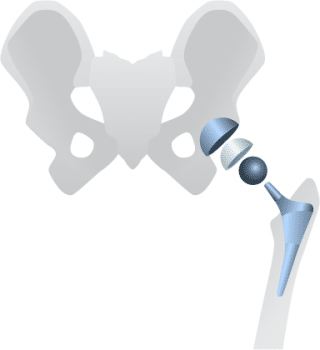
Possible metal-related complications can include:
- Metallosis – a buildup of metal debris from implants that can cause significant problems
- Pseudotumors
- Adverse tissue reactions around the implant
- Osteolysis – the destruction or disappearance of bone tissue
If you have been diagnosed with any of these metal-related conditions and require revision surgery, you may benefit from talking to a knowledgeable attorney. Weitz & Luxenberg’s attorneys have extensive experience in the area of faulty hip replacements and can advise you on your legal options.
Compensation for Smith & Nephew Modular SMF or Modular REDAPT Revision Hip Implants
Talking with an attorney familiar with hip implant cases can help you take into account other compensation to which you may be entitled, including:
Medical expenses
Pain and suffering
Lost earnings
Loss of consortium
If you have been harmed by Modular SMF or Modular REDAPT Revision hip implants, Weitz & Luxenberg’s experienced and compassionate attorneys are prepared to help you seek compensation for the harm you have suffered.
EMPERION Hip Problems
The Modular SMF and Modular REDAPT Revision announcement follows Weitz & Luxenberg scrutiny in 2015 of another modular implant made by the same company.
In July 2015, Weitz & Luxenberg announced the firm was investigating cases of device fracture with the Smith & Nephew EMPERION modular hip system after the Australian Orthopaedic Association found a significantly higher-than-average cumulative revision risk.
Weitz & Luxenberg continues to accept cases involving EMPERION hips along with the Modular SMF and Modular REDAPT Revision models.
Selecting an Attorney
Weitz & Luxenberg’s Drug and Medical Device Litigation attorneys – such as Ellen Relkin and Danielle Gold – combine broad experience in pharmaceutical and medical device litigations with personal attention to their clients.
Our clients have suffered life-altering injuries,” Ms. Gold said. “I am delighted and honored to be part of this caring team of supremely talented attorneys that offers the help these injured people urgently need.”
Nina Young turned to Weitz & Luxenberg after Young’s right and left hip implants failed.
“I chose Weitz & Luxenberg because the firm has a reputation for working really hard,” she said. “I felt that, if I was going to sue, I wanted a firm that had a lot of experience and a lot of resources. I wanted the big guns.”
“I liked that my Weitz & Luxenberg attorney and his team did not use an impersonal, one-size-fits-all formula for calculating how much money I should receive for my injury,” Young said. “The amount they said I could seek as compensation was calculated based on my unique circumstances.”
How Weitz & Luxenberg Can Help
For more than three decades, Weitz & Luxenberg has been dedicated to helping clients win cases. As a leader in medical device, asbestos, and personal injury cases, we have won more than $17 billion for our clients.
We are ready to help you. For your free consultation and more information on your legal options, please call us at (833) 977-3437 or complete our form on this page. Our client relations team will contact you shortly.
