Any product we buy or use may, in some way or form, come under scrutiny if it results in harm to people. It can be as simple as a toy malfunctioning or as serious as a defective medical device.
When we first think of product liability lawsuits, big-ticket items like cars may come to mind. In recent years, General Motors (GM) and its faulty ignition switch made headlines for months. GM’s recall affected millions of vehicles. In addition, questions regarding the company’s culpability reached as far as the Department of Justice and the federal court system.(1)

Aside from the automobile industry, the medical devices industry is responsible for significant harm to thousands of patients in some cases. For instance, manufacturers of hip, knee, and elbow implants; surgical mesh; and surgical instruments have been the target of multiple lawsuits in recent years.
People who undergo surgery and receive an implant of any kind face numerous risks. If the defective medical implant fails, it may require a patient to undergo additional revision, or corrective, surgery. Injuries and costs for these types of defective medical products can be significant, especially when pain and suffering are considered.
Product Liability
Whatever the product or its size, as consumers we have reasonable expectations about what that product is supposed to do. We expect products to be safe if we use them for their intended purpose.”
Even products that are not big-ticket items, such as a car or a medical implant, can cause severe injury. In reality, every single product we buy could potentially involve the question of product liability — talcum powder, tires, household appliances, football helmets, hair dye, and flooring products.
Defective football helmets could cause in severe head trauma. Even something as basic as talcum powder has been at the center of serious lawsuits in recent years, with some people claiming the powder caused ovarian cancer.
Whatever the product or its size, as consumers we have reasonable expectations about what that product is supposed to do. We expect products to be safe if we use them for their intended purpose.
Particularly concerning are products intended to keep us safe that turn out to be unsafe, such as smoke detectors that malfunction, fire alarms that fail to alert, and airbags that injure rather than protect. In recent years, you may recall Takata’s faulty airbags, which were installed in millions of vehicles nationwide.
In that instance, the faulty airbags could potentially explode upon inflation, and some did. The result: metal debris was sent flying, causing injury to those exposed. Some people even died from their injuries.
When we get injured or suffer harm while using or being exposed to a product, we may be able to seek compensation or restitution from the manufacturer. We can also ask for compensation from other contractors or businesses that played a role in making, distributing, and selling that product.
If you purchased a product that injured you or a loved one in some way, we encourage you to contact an experienced attorney, such as one of Weitz & Luxenberg’s product liability attorneys. With more than 30 years of handling large, often complex product liability cases, Weitz & Luxenberg can help you consider your legal options.
For a free initial consultation, we invite you to contact us by phone by calling (833) 977-3437, or you may complete one of our online forms. One of our representatives will be in touch with you shortly.

No federal product liability law exists protecting consumers from defective products. Instead, states create their own product liability statutes.
Basically, however, you may be able to file a product liability lawsuit if:(2)
Consumers should be able to expect products to do what manufacturers designed the products to do. In addition, if products provide instructions and safety precautions regarding how the product should be used, consumers should expect to remain safe while using that product accordingly.
The words “harm” and “injury” can refer to an obvious physical injury or death resulting from using or being exposed to a defective product. Sometimes the injury can be debilitating, such as a spinal cord injury resulting in paralysis, brain trauma, blindness, or scarring.(3)
If a vehicle is defective by design, it could tilt and roll over easily, seriously injuring not only everyone in that vehicle but also drivers and passengers in other cars. Potentially, that defect could be implicated in causing wrongful deaths and multiple life-threatening injuries.
Besides physical harm, people can suffer economic or emotional injury as well as damage to property. For example, a malfunctioning smoke detector could result in loss of property and lives.(4)
Whether your injury is physical, emotional, financial, or all of these, you may have grounds for a product liability lawsuit. To evaluate your situation further, we advise you to contact a law firm with experience handling product liability lawsuits, such as Weitz & Luxenberg.
For a free consultation and more information about your legal options, please contact us today.
Get a Free Case ReviewOur attorneys handle consumer fraud and product liability litigation on a large scale, whether it affects one person or thousands of consumers have been victimized. We also have a dedicated team of attorneys who specifically handle defective drugs and medical devices.
Whatever your specific circumstances, our attorneys have been trained to handle a wide variety of product liability lawsuits. We can assist you in evaluating your situation and the legal actions available to you.
Product liability lawsuits can be complex because they may involve multiple businesses or contractors:(5) (6)
Even before a product is manufactured, the design may be defective. Sometimes, there may be something inherently faulty about the product’s design.
Some defects may occur during the manufacturing or assembly process. The design of the product may be fine, but cracks or other defects could be introduced at the manufacturing plant. In the case of food items, for example, the manufacturing plant may contaminate the food product being prepared and packaged.
When it comes to marketing and promoting a product, the people involved may mislead consumers about its dangers or effectiveness, including medical devices or drugs. Improper labeling, incomplete or confusing instructions, or insufficient safety precautions and warnings can be considered grounds for a product liability lawsuit.

For over 30 years, Weitz & Luxenberg has handled cases involving everything from defective hip and knee implants to automobiles problems. Because we are a national firm, our attorneys are available across the country and are fully prepared to take on tough, large-scale cases involving industry giants.
Weitz & Luxenberg has stood up to Johnson & Johnson, Volkswagen, General Motors, Proctor & Gamble, Ford, and others who have created, manufactured, distributed, and sold defective products.
Weitz & Luxenberg was one of the first law firms to take legal action against Volkswagen for its consumer fraud debacle. Volkswagen had programmed its diesel vehicles to override emissions tests, testing lower for noxious emissions than the vehicles really generated.
Because our attorneys are dedicated to handling product liability cases, including defective drugs and medical devices, we have a solid history of winning. Some of our recent victories, and those where we combined forces with other law firms, include:
Weitz & Luxenberg is a product liability law firm with over three decades of representing clients harmed by defective products. In most cases, the product is inherently defective; for example, a poorly designed knee or hip implant.
With national recognition and 500 attorneys, paralegals, and support staff members, Weitz & Luxenberg is committed to helping people across the country who have been harmed by defective products and services. Over the years, we have assisted more than 50,000 clients across the United States, securing compensation of billions of dollars on their behalf.
We would feel privileged to assist you. For a free consultation and more information about your legal options, please contact us today.
(833) 977-3437If you have experienced harm or injury from a defective product, we encourage you to call us now at (833) 977-3437 or fill out the form on this web page, requesting to speak to one of our attorneys.
While our past record doesn’t guarantee future success, it is something you may want to consider when evaluating our experience.
In fact, prescription drugs accounted for $328.6 billion of national health-related spending in the United States in 2016. (1) According to the Centers for Disease Control and Prevention (CDC), “In 2015–2016, 45.8% of the U.S. population used prescription drugs in the past 30 days.” (2)
New drugs can bring new risks for people who take them. Adverse drug events (ADEs) can lead to serious medical complications or death.
In July 2018, a 9-year study (2006 to 2014) of the U.S. Food and Drug Administration (FDA) Adverse Events Reporting System database found 902,323 serious outcomes were reported to the FDA, including 244,408 deaths and 72,141 disabilities. (3)
New drugs must be approved by the FDA before they are sold. However, problems may still occur at any time — before, during, or after the approval process. In fact, many FDA-approved drugs have been linked with serious medical complications.
This is because drugs are tested only in a limited number of studies before being approved for the market. For this reason, it is important for patients and physicians to report drug adverse event to the FDA when such occur.
If you suffered severe complications or were hospitalized due to an adverse drug event, or if a relative died due to prescription drug-related complications, you may want to discuss your legal options with a knowledgeable attorney.
Weitz & Luxenberg may be able to help. We invite you to call us for a free consultation (833) 977-3437.
Consult your doctor before stopping any prescribed medication.
In 1938, Congress enacted the Food, Drug, and Cosmetic (FDC) Act. Since then, the drug approval process has continued to evolve.
We would feel privileged to assist you. For a free consultation and more information about your legal options, please contact us today.
(833) 977-3437In 1962, a sleeping pill called thalidomide, not approved for use in the U.S. due to efforts taken by Dr. Frances Kelsey at the FDA, resulted in thousands of babies being born with birth defects in Western Europe after their mothers had taken the drug during pregnancy. In response, Congress approved legislation requiring drug manufacturers to demonstrate to the FDA that any new drug is safe and effective for its intended use before selling it in the U.S. (4)
To secure FDA approval, drug manufacturers must conduct limited laboratory, animal, and clinical tests to determine how new drugs work and whether they are safe for use in humans.
A team at the FDA’s Center for Drug Evaluation and Research (CDER) composed of doctors, chemists, and other scientists reviews the evidence from these limited tests. If this group of FDA employees determines results presented from this testing demonstrate a level of safety and a measurable health benefit, the FDA may approve the drug. (5) In some cases, especially for particular classes of drugs, the FDA may approve a drug with the stipulation that additional postmarketing testing be conducted or that postmarketing safety monitoring of particular adverse events be performed by the manufacturer. (6) (7)

Sometimes the tests do not reveal all the potential risks of a new drug. In addition, in some cases, drug manufacturers fail to present all their evidence, submit erroneous or falsified information, fail to report adverse events or other study protocol violations, or fail to protect the safety of clinical trial patients. (8)
In addition, drug manufacturers may not communicate the risks of a drug to the public, even if the FDA discovers a problem at a clinical trial site during an inspection or after a medical journal publishes the results of a clinical trial. In fact, sometimes the problems can be substantial enough to cause or contribute to serious medical complications or even death.
Somewhere down the line, the FDA may require updated label instructions or warnings for complications, but, by then, the damage may have already been done. For some patients, the warning may come too late.
Adverse effects of medicines have spawned thousands of lawsuits in recent years.
For more than 30 years, Weitz & Luxenberg has been at the forefront of complex product liability-related litigation. Our attorneys have secured more than $17 billion in awards and settlements for clients.
Although past results are no guarantee of future success, Weitz & Luxenberg possesses the experience and resources necessary to pursue justice for clients harmed by the negligence of large drug companies.
If you have been injured by a defective drug, we can help you understand your legal options, beginning with a free consultation. Call us at (833) 977-3437 or fill out the form on this page. One of our representatives will be in touch with you shortly.
According to the U.S. Government Accountability Office, sales for 102 medical device companies in the United States increased 43% between 2005 and 2014.(2) Those numbers may give medical device manufacturers a reason to cheer. A good profit margin always boosts the morale of CEOs.
However, if you have been injured by a medical device, someone else’s profit margin may be the last thing on your mind. What you need right now is for someone to listen to you and take you seriously. You need someone who can provide you with dependable legal guidance and assistance. That is where Weitz & Luxenberg comes in.
If you have been injured by a faulty medical device, Weitz & Luxenberg may be able to help. Our firm has 30 years of experience in complex, large-scale, medical-related litigation. Over the years, we have represented hundreds of thousands of people. Our Weitz & Luxenberg lawyers are prepared to guide you through the legal process.
Weitz & Luxenberg is a national law firm. No matter where you live in the U.S. or which manufacturer made the medical device that harmed you, we are prepared to represent you and stand up to corporations or medical manufacturers of any size.
We do not back down from global medical manufacturers that have produced and distributed faulty, harmful medical devices. We stand by our clients, and we guarantee you can depend on us for solid, experienced guidance and legal counsel.

Not all medical devices are required to undergo comprehensive scientific and regulatory review before being marketed and sold in the United States. This is because a medical device manufacturer who believe that its device is “substantially equivalent” to a predicate device (one that has been cleared by the FDA or marketed before 1976) can apply to enter the U.S. market under the FDA 510(k) process. The 510(k) process bypasses the rigorous FDA Premarket Approval (PMA) process to evaluate the safety and effectiveness of new Class III medical devices. The purpose of a FDA 510(k) submission is to demonstrate that a device is “substantially equivalent” to a predicate device. Unlike the PMA process, which requires a manufacturer to present scientific evidence to assure that the device is safe and effective for its intended use(s), the 510(k) application submitter merely compares and contrasts its device with one or more predicate devices, explaining why any differences between the new and predicate device should not affect functioning. Clinical studies are usually not required for a 510(k) submission. Depending on the type of 510(k), the law gives the FDA either 30 or 90 days to decide “whether the device is equivalent to a device already” approved for distribution, ask further questions, or reject the application.(3)
For a free consultation and more information about your legal options, please contact us today.
Get a Free Case ReviewAlthough the FDA continues to monitor 510(k) medical devices after approval, such as through the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database,(4) manufacturers are primarily responsible for tracking, following-up on, and reporting adverse events occurring in patients using their products.
Sometimes, FDA approves a medical device and then growing clinical evidence demonstrates the serious harm the device causes when brought to market without being adequately tested by manufacturers for human safety.(5) (6)
If you have suffered medical complications linked to a faulty medical device, you have a right to seek compensation from the device manufacturer. At Weitz & Luxenberg, our defective medical device lawyers are here to help.
Currently, we are accepting clients who have been harmed by certain types of:
If you have been injured by a defective medical device, Weitz & Luxenberg wants to hear from you. We offer a free consultation. One of our attorneys can help you review and understand your legal options.
Although many of the medical devices we use at home, buy in stores, or see in a medical facility have been approved by the FDA, that does not necessarily mean they are safe. Manufacturers frequently issue medical device recalls for products that were approved by the FDA.
You may hear about defective medical device recalls by watching or reading the news. In addition, you can search the FDA’s database for the most updated information.
Medical Device Recalls
Although many of the medical devices we use at home, buy in stores, or see in a medical facility have been approved by the FDA, that does not necessarily mean they are safe.”
According to the FDA, the agency “posts summaries of information about the most serious medical device recalls.” Devices are included in those posts when the FDA believes that there is the possibility “they could cause serious health problems or death.”(7)
Whether or not a manufacturer has recalled a medical device, you still have the right to look into taking legal action if you have been injured by a faulty medical device. At Weitz & Luxenberg, we stay on top of all significant FDA medical device safety announcements and remain informed about all related legal proceedings.
If you believe you have suffered medical complications due to any kind of medical device, we encourage you to call us for a free consultation. We will be happy to discuss the legal options available to you.
Total hip replacement (arthroplasty) is an orthopedic intervention. Some hip systems have been made out of all-metal materials.(8) It is estimated that more than one million people around the world have metal-on-metal (MoM) hip implants.(9)
Several models of all-metal hip implants have shown a higher-than-anticipated failure rate, leading to serious injuries or medical complications for patients. In response, the FDA has issued a number of safety communications and several recalls have been initiated.(10) (11)
Corrosion or wear between metal-on-metal parts can release chromium, cobalt, and titanium ions into the body. Metal debris can damage tissue and lead to a condition called metallosis – which can starve tissue of oxygen and lead to cell death, potentially causing a host of problems.(12) (13) Other complications from defective metal-on-metal hip implants may include:
Correcting these issues often requires revision surgery, which subjects a patient to further risks and possible complications.
In recent years, a number of patients have experienced severe complications following metal-on-metal hip replacement surgery. In response, Weitz & Luxenberg has taken legal action against hip implant manufacturers.

In 2014, Weitz & Luxenberg Practice Group Co-Chair Ellen Relkin served a key role in multidistrict litigation against DePuy Orthopaedics, a subsidiary of Johnson & Johnson, regarding its defective articular surface replacement (ASR) metal-on-metal hip implant. Ms. Relkin helped secure a $2.5 billion settlement against DePuy on behalf of patients who had received the faulty implant.
More recently, in 2016, juries in separate trials sided against DePuy regarding its Pinnacle hip implant when it was used in a metal-on-metal configuration. They have awarded more than one billion dollars across multiple suits to plaintiffs related to defective Pinnacle hip implants. DePuy faces thousands of additional lawsuits pending nationwide regarding its defective Pinnacle hip.(17)
A growing body of medical literature is linking the Smith & Nephew EMPERION hip implant system to sudden fractures of the implant. In addition to discussing high rates of failure due to implant fracture, the Australian Joint Registry has identified the Emperion as having a significantly higher percentage of revisions than other primary total hip replacements, with more than two times the risk of revision occurring with the Emperion.(18) (19) (20) (21)
Weitz & Luxenberg continues to accept cases involving faulty Smith & Nephew EMPERION hips along with Modular SMF and Modular REDAPT models, the latter of which have been recalled due to potential for higher risk of revision.(22) If you suffered complications after receiving one of these Smith & Nephew hip implants, we encourage you to contact us.

In November 2014, and then extended in December 2016, Stryker Orthopaedics agreed to a $1 billion settlement with patients implanted with its Rejuvenate and ABG II modular neck stems. Stryker “expects the majority of the payments under the expanded settlement agreement will be made by the close of 2017.”(23)
Weitz & Luxenberg’s Ellen Relkin is credited with playing a key role in this settlement.
In August 2016, Stryker recalled certain lots and sizes of its LFIT Anatomic Cobalt Chromium V40 Femoral Heads (LFIT V40 femoral heads) due to the company receiving complaints of taper lock failure.(24) Taper locks are located at the junction where the femoral head attaches to the stem of the hip implant.
In April 2017, Panel on Multidistrict Litigation agreed to consolidate more than two dozen lawsuits pending against Stryker into a multidistrict litigation in the District of Massachusetts.(25)
Plaintiffs claim the LFIT V40 femoral heads can release toxic and corrosive cobalt into people implanted with the devices, potentially necessitating revision surgery. The complaints state the corrosive effects can be severe enough to cause the femoral heads to break off and become separated from the rest of the implant.(26)
In 2016, Wright Medical Group agreed to settle more than 1,200 lawsuits over its metal-on-metal Conserve, Lineage, and Dynasty hip implants.(27) As co-lead counsel, Weitz & Luxenberg’s Ellen Relkin again played a role in crafting the $240 million settlement agreement.
A Class 1 recall is the most serious. The FDA issues a Class 1 recall in situations “in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”(30)
In 2015, MicroPort’s Profemur Neck Varus/Valgus CoCr 8 Degree modular neck was voluntarily recalled, with the FDA classifying this as a Class 1 recall. Originally, Wright Medical designed and manufactured this modular neck, but Wright’s OrthoRecon Business was acquired by MicroPort Scientific Corporation in June, 2013.(28) (29)
A Class 1 recall is the most serious. The FDA issues a Class 1 recall in situations “in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”(30)
The FDA’s safety alert warned that the modular neck could fracture, requiring emergency revision surgery. The FDA said a sudden fracture “could lead to neurovascular damage, hematoma, hemorrhage, and even death.”(31)
If you have been injured by a Wright Medical Conserve, Lineage, or Dynasty hip implant or by a Profemur Neck Varus/Valgus CoCr 8 Degree modular neck requiring revision surgery, you may want to consult an experienced attorney. The attorneys at Weitz & Luxenberg offer a free consultation. We can help you consider your legal options.
Following knee implant surgery, some pain is to be expected. However, some problems and complications may be serious.(32)
In 2010, Zimmer recalled components for several versions of the company’s NexGen Complete Knee Solution.(33) (34) As of June 2017, cases are still pending in a multidistrict litigation in Illinois.(35)
More recently, in 2015, Zimmer Biomet voluntarily recalled more than 11,000 components used in the Zimmer Persona Knee Personalized Knee System after complaints of loosening and radiolucent lines. The FDA categorized the recall as Class 2.(36)
If you had a Zimmer Persona Knee Personalized Knee System implanted and required revision surgery, we encourage you to call Weitz & Luxenberg. We offer a free consultation and can offer guidance about your legal options. Call us if you have had any of these complications:
The Smith & Nephew Journey Bi-cruciate Stabilized (BCS) Knee replacement system (Journey I BCS Knee System) received 510(k) approval from the FDA in 2005. However, in June 2018, the manufacturer issued an Urgent Field Safety Notice announcing the voluntary removal of the system’s femoral component from the market due to a higher than expected risk of revision in patients implanted with the knee replacement. (37)
This action was followed by the FDA classifying the recall as a Class II device recall for the Journey BCS Knee System on October 1, 2018. (38) The FDA’s recall was based upon device failure data.
Device failures were noted during the postmarket tracking and follow-up process, with data indicating revision surgery rates with the first generation Journey BCS Knee over 1.5 times higher than other knee replacement devices. These high revision rates were found in the National Joint Registry of England, Wales, and Northern Ireland and the Australian Orthopaedic Association National Joint Replacement Registry. (39)
The most common reason for device failure was component loosening. (40) According to the Hospital for Special Surgery, indications of total knee replacement component loosening include:
When components in knee devices loosen, revision surgery is often required. Revision surgeries are expensive, are longer and more complex surgeries than initial knee replacement surgery, and have greater risk for complications. (42)
Follow-up care and physical therapy are typically needed. You may lose additional time from work or have complications, which can cost you even more money.
So, if you or a loved one had a Smith & Nephew Journey BCS Knee replacement system implanted that required revision surgery, or have been advised by your doctor that you need revision surgery due to femoral component loosening, we encourage you to contact Weitz & Luxenberg. The initial consultation is free, and we would be happy to provide guidance about your legal options.
The complications you may suffer from after undergoing shoulder implant surgery with a defective shoulder device are similar to those of defective knee implants. These complications include:(43)
If you experience unexpected or severe complications, let your health care provider know right away. Sometimes complications may require a revision, or corrective, surgery, or additional treatment.
According to the FDA, one million hernia repair procedures are performed each year in the United States.(44) Although the FDA has approved the surgical hernia mesh devices on the market today, the agency has issued warnings about possible complications, and notes that several meshes have been recalled.(45)
Complications of surgical mesh used in hernia repair include:(46)
Weitz & Luxenberg is accepting cases of complications occurring with these surgical meshes used in hernia repair:
Some complications reported with these brands include:(47) (48) (49) (50)
If you have suffered medical device complications requiring hospitalization or corrective surgery due to problems with the mesh used in your hernia repair, you may be entitled to compensation for medical costs and other expenses. Weitz & Luxenberg invites you to contact the firm for a free consultation to help you understand your legal options.
Hear From Our Clients
You have helped me to close a very difficult chapter in my life, both emotionally and financially, and I am incredibly grateful to you. About a day after I received my check and had deposited it, the reality of what you and your firm accomplished on my behalf finally sunk in — I cried. I feel victorious and that in some small way justice has prevailed for the little guy (gal) over the corporate giant. Thanks for leveling the playing field.”
Leslie H.
We have represented thousands of clients across the country, winning more than $17 billion on their behalf.”
As a national law firm with more than 30 years of experience winning complex, large-scale, defective medical device lawsuits, Weitz & Luxenberg is prepared to take on the challenging cases. We have represented thousands of clients across the country, winning more than $17 billion on their behalf.
If you have been injured by a medical device, we urge you to call us now. We offer a free consultation and can help you consider your legal options.
You can reach us by phone at (833) 977-3437 or fill out the form on this page. One of our representatives will contact you shortly.
Although a past record does not guarantee future success, Weitz & Luxenberg has the experience and resources necessary to stand up to the large manufacturers of defective medical devices.
Replacement surgeries most commonly involve the hip or knee, although most joints in the body can be replaced including shoulders, wrists and elbows.
For hip replacement alone, more than 450,000 surgeries are performed annually. Ideally, patients today should expect a hip or knee replacement to last for 20 or more years.
Complications, however, do occur. In fact, for joint replacement in general, the percentage of people experiencing complications may be as much as 40 percent.
For a free consultation and more information about your legal options, please contact us today.
Get a Free Case ReviewJoint replacement surgery, also known as arthroplasty, involves removing the worn or damaged cartilage from both sides of the joint, followed by resurfacing the joint with a replacement implant. The implant is designed to mimic a normal joint and should allow patients to resume a high degree of normal activity within a few months.
Some pain following surgery is to be expected. However, if it is severe, or is manageable at first but worsens over time, that is a concern. It could mean that the joint replacement is failing.”
Historically, the use of hip implants was limited to patients who were older. These patients were less active, had suffered a hip fracture or experienced severe arthritic hip conditions.
Currently, orthopedic surgeons have been offering hip replacement options to patients as young as 55, perhaps younger. Implants are being made of a variety of materials, including combinations of metal, polyethylene and ceramic.
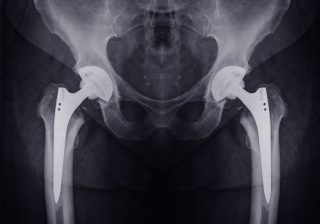
Herein lies good news and bad. With the demand for products and devices suitable for younger, sometimes far more active patients, manufacturers have been aiming to create stronger, more durable, more flexible designs. In some cases, this has led to defective products that have been inadequately tested and researched.
There are almost as many complications as there are joints in the body.
In many cases, you may not be aware what type of implant you received. A good lawyer can determine if you have been subjected to a defective device. With your permission, an experienced lawyer can get access to your records and help you understand your options.
Choosing the right attorney may feel overwhelming when you are suffering from a joint replacement failure. To select the firm that will best represent you, you need to ask the right questions. What experience can this firm offer me? What is this firm’s philosophy toward working with its clients?
Weitz & Luxenberg stands out for its success in winning complicated medical lawsuits. We represent tens of thousands of individuals, consistently offering superior results. You can be assured that if you select Weitz & Luxenberg, we will fight with you and for you every step of the way.
We would feel privileged to assist you. For a free consultation and more information about your legal options, please contact us today.
(833) 977-3437As a nationally recognized personal injury law firm, Weitz & Luxenberg is committed to helping clients win cases. For more than 25 years, we have dedicated ourselves to holding irresponsible practitioners accountable, and we have won $17 billion for our clients.
We would feel privileged to assist you. For a free consultation and more information about your legal options, please call us at (833) 977-3437. If you prefer, you can complete our form, and our client relations representative will contact you shortly.
Weitz & Luxenberg is an advocate and leader in the fight to protect the public from contaminated drinking water. A recent study of public water supplies found unsafe levels of chemicals in the water consumed every day by 6 million people.(1) Unfortunately, that number represents only the tip of the iceberg when you factor in the number of people whose water was not tested as part of the study, including those who consume contaminated water from private wells, or people whose water is at risk of being contaminated.
The fact is, many in the U.S. assume their drinking water is safe only to learn later they have been consuming toxic chemicals due to inadequate pollution controls and hazardous waste disposal practices. The results for a community like Flint, Michigan, for example, where residents were told their water was safe even after it was knowingly switched to an unsafe source, have been devastating. In other communities, like Hoosick Falls, New York, residents only recently learned that they have been consuming a tasteless, odorless toxic chemical for decades.
Weitz & Luxenberg brought actions on behalf of municipal water providers against various international oil companies that contaminated groundwater with the gasoline additive methyl tertiary-butyl ether (MTBE). MTBE is known to cause cancer when consumed by humans. Due to the contamination, the municipal water providers were unable to deliver safe, clean water to residents for consumption and other general uses.
Weitz & Luxenberg served as co-liaison counsel in the MTBE litigation, which revealed pollution of water wells and reservoirs in 17 states. Ultimately, Weitz & Luxenberg secured a $423-million settlement on behalf of numerous public water supply companies that sought to bring clean water to their communities.
The firm’s environmental unit has expanded into the Environmental, Toxic Tort & Consumer Protection Unit and has continued to take a leading position litigating against polluters. The firm continues to represent municipal water providers whose production wells have become polluted. The unit also works with communities, such as Flint, Michigan, and Hoosick Falls, New York, or with individuals directly exposed to contaminants in their drinking water.
Robin Greenwald heads the firm’s Environmental, Toxic Tort & Consumer Protection Unit. Prior to joining Weitz & Luxenberg in 2005, Ms. Greenwald served as a senior attorney in the U.S. Department of Justice. Ms. Greenwald began her career in law as an assistant U.S. Attorney for the Eastern District of New York. She later became assistant chief of the Environmental Crimes Section of the Department of Justice, in charge of Legislation, Policy and Special Litigation.
From there, Ms. Greenwald was appointed as General Counsel for the Inspector General of the U.S. Department of the Interior. She then served as executive director of an international not-for-profit water protection organization and as a clinical professor of law at Rutgers Law School.
Federal judges have consistently recognized Ms. Greenwald’s knowledge and expertise in environmental law. She has served as plaintiffs’ liaison counsel in In Re: Methyl Tertiary Butyl Ether (”MTBE”) Products Liability Litigation (MDL 1358) for the past decade, and she was named a member of the Plaintiffs’ Steering Committee for the BP Oil Spill litigation (MDL 2179), Volkswagen “Clean Diesel” Marketing litigation (MDL 2672), and the Southern California Gas Leak litigation (JCCP No. 4861).
Have you been notified that your drinking water is contaminated? Contact us today.
Get a Free Case ReviewA team of eight attorneys work within the unit and they possess, collectively, nearly a century of legal experience. The unit’s attorneys work closely with our clients and experts within the field and have developed an extensive knowledge of relevant information and the ability to effectively convey that information to the court. Understanding the toxicology of a chemical and the hydrogeology relevant to transporting a contaminant in groundwater from a release point to a drinking water production well, as well as having the ability to present that information in a concise and coherent manner, requires a deft touch that only comes with experience and ability.
Hear From Our Clients
The firm genuinely cared about my family and diligently worked hard on our case and saw it through to the end.”
Wendy
Water providers, communities, and individual victims of water contamination can seek compensation through the legal system. Weitz & Luxenberg firmly believes that the party responsible for the contamination should shoulder the obligation and the cost for all injuries arising from pollution in drinking water.
Attorneys with the environmental unit also are currently working with the Attorneys General of Vermont and Rhode Island in connection with statewide groundwater contamination from MTBE.
Weitz & Luxenberg hosts meetings in communities affected by environmental pollution. We have met with families in New York, Pennsylvania, New Hampshire, and Michigan to listen to their stories and provide updates on our investigations into water contamination.
For more information about what we have learned so far in our investigations or to discuss your legal options, please call (833) 977-3437 or fill out our form.
We understand your concerns and anger over water contamination in your community, and we want to help. There is no charge for our legal consultations.
Ninety percent of Americans get their water from public drinking water systems regulated by the U.S. Environmental Protection Agency (EPA).(2) Public drinking water systems can be publicly or privately owned. The EPA regulates the drinking water quality and sets the maximum concentration levels for water chemicals and pollutants.(3)
Surface water — water that collects in streams, rivers, lakes, and reservoirs. Surface water systems extract water, treat it, and deliver it to homes.
Groundwater — water that collects in pores and spaces within rocks and in underground aquifers. Groundwater systems drill wells and pump water to the surface. They do not always treat the water before delivering it to homes.(4)

The U.S. has about 155,000 public water systems. The EPA identifies these water systems based on how many people they serve, where their water comes from, and whether they serve the same customers all year or less frequently.
The EPA classifies public water systems into three types:
More than 286 million Americans get their tap water from a community water system, according to the Centers for Disease Control and Prevention (CDC). Most community water systems use groundwater, but more people get their water from community water systems that use surface water. Eight percent of U.S. community water systems provide water to 82% of the U.S. population through large municipal water systems.(6)
In 1974, Congress passed the Safe Drinking Water Act, which sought to protect the nation’s public drinking water supply.
The law gives the EPA authority to determine the standards for drinking water quality and to work with the states and water suppliers who apply those standards.
Congress amended the law in 1986 and 1996 to protect drinking water and its sources, including rivers, lakes, reservoirs, springs, and groundwater wells.(7)
Contamination of public water systems can come from many sources. Contaminants can be natural or human-induced. Industrial discharges, urban activities, agriculture, and disposal of waste can all affect water quality. So can leaking fuel tanks, toxic chemical spills, and pesticides and fertilizers applied to lawns and crops. (8)
According to the CDC, the most common sources of contaminants are:

PFOA and PFOS are part of the group of chemicals called perfluoroalkyl substances (PFAS). These chemicals have been used in numerous industrial processes and for fighting fires at airfields.
The EPA issued lifetime health advisories for PFOA and PFOS in 2016. The agency says it develops health advisories to provide information on contaminants that can cause health reactions in humans and are known or anticipated to occur in drinking water.(10)
MTBE is a flammable, colorless liquid added to gasoline to help it burn better. For most people, water contamination is the most likely source of MTBE exposure. MTBE can contaminate water through gasoline spills and leaking gas storage tanks and pipelines.
If gasoline is spilled onto the ground or leaks out of underground storage tanks, MTBE is more likely to contaminate drinking water because it dissolves easily and can travel faster and farther through groundwater than other components of gasoline. It also can remain in underground water for a long time.(11)
If a water system becomes contaminated, such as in Hoosick Falls, New York, anyone who drinks the water may be at risk of developing adverse health effects.
Weitz & Luxenberg has brought legal actions on behalf of individuals who have suffered a specific injury from exposure to hazardous chemicals in their drinking water or environment. These cases remain extremely complicated and, unfortunately, limited.
Despite what one might see as an obvious link between contaminated drinking water and an unexplained illness, in most cases, the law limits recovery to cases where we can demonstrate with expert testimony a link between a specific chemical and a specific injury and show that the individual was exposed to that chemical at a level sufficient to result in the illness.
Although an injury or death wasn’t intended, people and businesses may be legally responsible to accident victims. If another person or corporation is responsible for an accident hurting or killing someone, Weitz & Luxenberg may be able to help you obtain compensation.
When people experience a traumatic brain injury in an accident, they may need financial help to cope with medical and living needs.
Weitz & Luxenberg’s expert airline law attorney obtained a major settlement for a woman who was injured when a suitcase fell from an overhead bin in an airplane and hit her on the head. The firm also secured millions for a man who fell from an apartment building’s second-story walkway.

Thousands of workers are killed on the job every year. There were 5,486 fatal occupational work injuries in 2022, according to federal government statistics. The government calculated 3.7 fatal work injuries for every 100,000 full-time equivalent workers. (1)
The Occupational Safety and Health Administration (OSHA) keeps records of all reported on-the-job deaths. Some examples from 2022 and 2023:
Data from 2022 indicates most fatal injuries at work involved transportation: 1,620 deaths resulted from transportation and materials moving incidents. There were 865 fatalities due to work related falls, specifically falls from higher levels. Another 738 people died from contact with objects or equipment. (3)
Need assistance after you or your loved one were injured in an accident? Speak to a top attorney today for a free consultation.
(833) 977-3437Workplace deaths can be caused in a myriad of ways, some very unexpected. For example, between 2019 and 2023, 19 people died on the job in incidents involving insects. Most of these were from bee stings. (4)
Occupations with high numbers of on-the-job fatalities included transportation and materials moving, construction and extraction, protective services, installation, maintenance, and repairs. The farming, fishing, and forestry occupational category had the highest fatality rate, with 23.5 fatalities per 100,000 workers in 2022. (5)

Supermarkets and malls can be hazardous to the public. Accidental injuries and even deaths can happen in those establishments if the proprietors aren’t careful in making their stores safe for customers.
In 2022, there were 174 preventable deaths in the retail industry, according to the National Safety Council. (6)
One example of retail incidents involves vehicle crashes. Data shows over 100 vehicles per day crash into retail and commercial buildings, resulting in 16,000 injuries and 2,600 fatalities annually, according to the Storefront Safety Council. (7) The preventative measures retail establishments are urged to implement include ensuring parking and pick-up/drop-off points are located away from buildings. (8)
Even grocery store shopping carts pose a substantial hazard to children. One study found over 24,000 children younger than 15 are treated annually in U.S. hospital emergency rooms for injuries related to shopping carts. (9) In 78.1% of these cases, the injuries were to the head region.
Slips, trips, and falls are a leading cause of injuries, especially in parking lots. There are steps to take to help prevent such injuries. These are some recommendations: (10) (11)

According to the National Highway Traffic Safety Administration (NHTSA), 42,795 people died in motor vehicle crashes in 2022. This put the U.S. crash fatality rate at 1.35 per 100 million vehicle miles traveled (VMT). Also, 23 states were expected to have increases in traffic fatalities in 2022, according to NHTSA. (12) (13)
Were you or a loved one injured in an accident? You may be eligible for compensation.
Get a Free Case ReviewAmong other NHTSA findings, these categories showed fatality increases in 2022 as compared with the first half of 2021: (14)
Motor vehicle crashes were a leading cause of death for children age 14 and younger in 2021, at a total of 1,184 deaths. “An average of 3 children were killed and an estimated 445 children were injured every day in traffic crashes in 2021,” according to NHTSA data. (15)
When you travel on any highway, you are trusting people you don’t know to exercise the same caution as you do — so everyone arrives safely at their destinations. But that trust isn’t always rewarded.
According to government data, over 5,788 people were killed in crashes involving large trucks in the United States in 2021, a 17% increase from 2020. Of these victims killed, 72% were occupants of other vehicles. (16)
Truck drivers involved in crashes were less likely to have a history of license suspensions and lower alcohol levels than other drivers. However, they were more likely to have been involved in more vehicle crashes than drivers of other types of vehicle. (17)
On the other end, motorcyclists make up 14% of fatalities. The total number of motorcyclist fatalities in 2021 went up by 8 % to 5,932. (18)
The federal government estimates motor vehicle crashes cost $340 billion in lost productivity, workplace losses, legal and court expenses, medical costs, emergency medical services, insurance administration, congestion, and property damage. (19)
Alcohol contributed to the cost estimate at $68.9 billion. It was followed by speeding at $46 billion. (20)
In 2021, 40% of fatal crashes happened in rural areas and 60% in urban areas. It’s estimated 20% of the population lives in rural areas. Overall, rural areas saw a 5% increase in traffic fatalities, while urban areas saw a 14% increase. (21)
But doctors and medical science are far from perfect. And although physicians routinely save and dramatically improve their patients’ lives, sometimes they have the opposite effect.
Bad decisions, mistakes, and failures to properly diagnose and treat patients can devastate entire families. These errors can be serious; they can leave patients permanently disabled or dead.
If you or a loved one has been harmed by medical malpractice, Weitz & Luxenberg can help you receive compensation. Weitz & Luxenberg has the experience and expertise to pursue cases against careless medical providers in an era of increasing legal obstacles to such litigations.
Medical mistakes are far more common than you may think.
A 2016 study by researchers at Johns Hopkins University School of Medicine found that medical errors are the third leading cause of death in the United States.(1) The study analyzed death rate data over eight years and found more than 250,000 deaths a year can be attributed to medical errors, ahead of the Centers for Disease Control and Prevention’s third-place cause, respiratory disease, which kills almost 150,000 people annually. The top two causes of death were identified as heart disease and cancer, with about 611,000 deaths and 585,000 deaths respectively.(2)
The authors of the study criticized the CDC for failing to create a standardized method for keeping track of fatal medical mistakes. Johns Hopkins surgery Professor Martin Makary says the U.S. is using a system adopted in 1949 when officials didn’t fully appreciate that medical mistakes could kill.(2) Because deaths from medical errors are not tracked nationally, Makary says, the issue doesn’t get the attention it should.(2)
In a letter to the CDC, the authors maintained that tracking deaths from medical errors could lead to research that would reduce such errors by advancing technology that “reduces harmful and unwarranted variation” in medical care.(3)
Have you been harmed by medical malpractice? We may be able to help. Contact us now for a free consultation.
Get a Free Case Review“Reducing costly medical errors is critical towards the important goal of creating a safer, more reliable health care system,” the letter concluded. “Measuring and understanding the problem is the first step.”(3)
Makary was involved in another study in 2012 at Johns Hopkins that found surgeons leave foreign objects such as a sponges inside patients’ bodies after operations 39 times a week, perform the wrong procedures 20 times a week, and operate on the wrong part of the body 20 times a week. All told, researchers in that study estimated 80,000 of these totally preventable incidents occurred between 1990 and 2010, a rate of about 4,000 a year.(4)
But even with deaths from medical errors being so prevalent, researchers have found that the rate of medical malpractice claims has dropped drastically in the last 20 years, according to the findings of a study at Brigham and Women’s Hospital. In that time, the overall rate of claims paid dropped by more than 55%. The reduction in claims varied widely, depending on the specialty, with pediatricians having the steepest drop at more than 75% and cardiologists having the smallest dip, 13.5%.(5)
The study also ranked the medical mishaps involved in the paid claims, with the most common being error in diagnosis, which accounted for almost 32% of the claims. Next were errors related to surgery at nearly 27%, followed by errors related to medication or treatment at 24.5%.(5)
32%
Error in diagnosis
27%
Errors related to surgery
24.5%
But while the rate of claims paid has dropped significantly, the amount of money awarded when claims are paid has increased, researchers found. The same study found the number of awards over $1 million on the rise.(5) The average payout for successful claims increased by about 23% to about $353,000 between 2009 and 2014.(6)
The lead researcher, Dr. Adam Schaffer, who teaches at Harvard Medical School, told CBS News that laws aimed at curbing medical malpractice litigations could make it more difficult to find attorneys to bring such claims, which could explain the drop in paid claims. Other reforms have served to screen out claims before they get to court.(6) Those cases that make it through the process could therefore be more likely to result in larger awards.(6)
Need legal assistance after being injured by medical malpractice? Speak to an experienced attorney today for a free consultation.
(833) 977-3437Another study found that a very small number of physicians is responsible for a large portion of malpractice awards. That study, published in the Journal of Patient Safety, found that less than 2% of doctors are responsible for half of malpractice payouts reported to the National Practitioner Databank over 25 years. And the likelihood a physician would have a malpractice payout increased with each malpractice award. In other words, once a doctor was the subject of a successful malpractice claim, the odds would increase for that doctor having to make future malpractice payouts.(7)
In order to prove a medical malpractice case, a patient has to show four things:
The concept of medical malpractice dates back to ancient Rome and was introduced into Europe around 1200 AD.(7) The first medical malpractice in the United States began appearing regularly in the 19th century, but cases were rare and had little impact on medicine until the 1960s.(8)
Observant patients may help reduce incidents of malpractice.
A study conducted at Vanderbilt University Medical Center found that surgeons who had the most complaints from patients and family about rude and disrespectful behavior had higher rates of bad outcomes from surgery.(9) That’s because those doctors’ personalities not only rub patients and families the wrong way, but they make work unpleasant for colleagues in the operating room and make it harder for other members of the surgical team to work.(8) Patients receiving care from surgeons with high numbers of complaints saw almost 14% more surgical and medical complications in the 30 days after their operations.(9) If you encounter a rude surgeon, you should not hesitate to speak up. The same goes for family members. Chances are people who work with that surgeon may find him insufferable, as well, and may be afraid to point out potential problems.
One of the researchers estimated that these surly surgeons could be responsible for more than 350,000 post-surgical complications, including infections, every year.(9)